How Many Valence Electrons Do the Noble Gases Have
Radium Ra has two valence electrons. Neon has 8 Valence electrons.

Why Are Atoms With 8 Valence Electrons So Stable Electron Configuration Electrons Covalent Bonding
The octet rule determines the number of valence electrons in the noble gases excluding He.

. Experts are tested by Chegg as specialists in their subject area. This problem has been solved. How many valence electrons do noble gases have.
This is the most stable arrangement of electrons so noble gases rarely react with other elements and form compounds. Why are noble gases the least reactive group. 22 Does CL have 8 valence electrons.
With regards to noble gases they have the outer s p orbitals full - 8 electrons. How reactive are noble gases. In summary the noble gases have 8 valence electrons each except for helium which has 2 valence electrons.
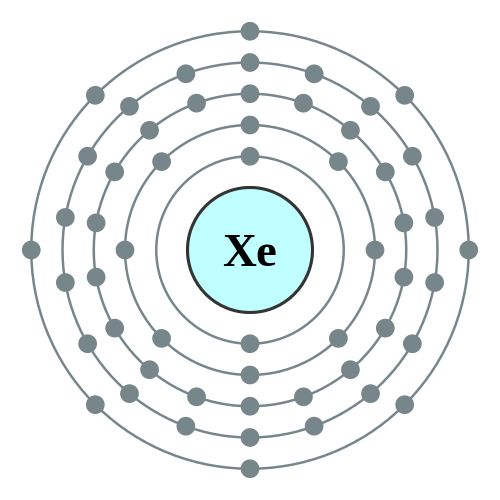
Valence electrons are placed in the outermost orbital of an atom. I - is isoelectric with xenon same number of electrons so yes it would have 8 valence electrons. Of these the three outermost electrons in the sixth shell are valence electrons.
Neon like all noble gases has a full valence shell. The last and only level of heliums electronic configuration is 1s2 and therefore He has 2 valence electrons. A radium atom has two valence electrons.
Inert gases have 2 or 8 electrons in the. Because they have the full 8 valence electrons in their outer energy levels meaning that they are reluctant to gain or lose a. 24 How many electrons does chlorine shell have.
Valence electrons in Sodium Na 1. Noble gases are typically nonreactive. How many valence electrons do the noble gases possess in the second period and below in the periodic table.
The noble gases are colorless odorless tasteless and nonflammable under standard conditions. As for the symbol remember that an atom wants 8 electrons in its outer ring if possible. Valence electrons in Carbon C 4.
What is the number of valence electrons in Radium. Noble gases are the least reactive of all elements. Elements belonging to this group are helium neon argon krypton xenon and radon.
The noble gases are the only non-transition-metal elements that have eight valence electrons in their neutral ground state configuration but atoms of other elements can produce a full octet -- an outer shell with eight electrons -- by gaining or losing electrons. All noble gases has 8 valence electrons except HeHelium wala nang iba which is 2 valence electrons. How many valence electrons do noble gases have.
See the answer See the answer See the answer done loading. 25 What are the valence electrons of all elements. View the full answer.
8 How many valence electrons are in BA. These electrons combine with other atoms to form molecules. Noble gases have 8 electrons.
Valence electrons are the electrons in the outer orbitals the s and p. Lewis Dot Symbol Of XenonXe. See answer 1 All of the noble gases have eight valence electrons with the exception of Helium which has two.
How many valence electrons do noble gases have. 1 2 7 6 8. Noble gases have the general electronic configuration ns2np6.
Noble gases have eight electrons in their outermost shell except in the case of helium which has two. Valence electrons in Nitrogen N 5. Valence electron is an outer shell electron that is associated with an atom and that can participate in the formation of a chemical bond if the outer shell is not closed in a single covalent bond both atoms contribute one valence electron in.
Helium has two electrons in total and according to the aufbau principle it adopts the electronic configuration 1s2. 1 or 2 2 or 8 2 or 3 8 Get the answers you need now. True there are 8 electrons in the valence level making it full True or False.
23 Does chlorine have 7 valence electrons. Thats because they have eight valence electrons which fill their outer energy level. Valence electrons in Neon Ne 8.
Valence electrons in Oxygen O 6. 20 How many valence electron does bromine have. Noble gases also called inert gases belong to the group 18 of the periodic table.
Valence electrons in Noble Gases. 21 How many valence electrons does neon have. Valence electrons in Boron B 3.
Valence electrons in Beryllium Be 2. Valence electrons in Fluorine F 7. This is the halogen group and all of them have 7 valence electrons hence the column.
100 4 ratings Answer E 8 electrons Neon like all noble. This means it has two electrons in s orbitals with a principal quantum number of 1. They do however have some cool applications eg.
They can be as many as 8 and as few as 0. Draw the Lewis dot symbol of - 10511134 marlenevalliente marlenevalliente 07022021 Chemistry Senior High School answered 4. Valence electrons in Magnesium.
The Noble Gases have electronegatives False The Noble Gases have very low chemical reactivity. There are six naturally occuring noble gases are helium He neon Ne argon Ar Krypton Kr xenon Xe and the radioactive radon Rn. Sulphur has 6 valence electrons 3.
Nitrogen has 5 valence electrons 2. Broadly chemical bonding is classified into two categories based on how the valence electrons of an atom interact with the valence electrons of other atoms. How many valence electrons do noble gases have.
A thallium atom has 81 electrons arranged in the electron configuration Xe4f 14 5d 10 6s 2 6p 1. Role of Valence Electrons in Bond Formation. Does boron have 3 or 12 valence electrons.
Noble gases like NeAr and Kr has 8 valence electrons. Stan SB19 for better gradescharaught. To do this it will more likely gain 1 than lose 7 so it will be a Chlorine ion with an extra electron.
We review their content and use your feedback to keep the quality high. While we can do an electron configuration chart to determine the location of all 88 of radiums electrons in. Chlorine is in column 7 immediately before the noble gases.

How To Find Valence Electrons Chemistry Classroom Science Chemistry Chemistry

How To Find Valence Electrons Chemical Equation Electron Configuration Ionization Energy
More Cheaty Revision Actual Xenon Compounds Electrons Element Chemistry Electron Configuration

Science Coverage How Many Valence Electrons Does Argon Ar Have Element Chemistry Electrons Argon
Comments
Post a Comment